Introduction. Type I Gaucher disease (GD1) has been associated with increased cancer risk in the International Collaborative Gaucher Group (ICGG) Registry. Particularly, it is well recognized that the risk for multiple myeloma (MM) in affected individuals may be about 9 times higher than expected in the general US populations (Rosenbloom et al, 2022). Pathogenetic relationship between GD1 and MM is still unclear. It was hypothesized that accumulation of glucosylceramide in the macrophages leads to their activation and chronic immune stimulation favouring polyclonal gammopathies. In this scenario plasma cell clone selection could be promoted and subsequent MGUS/MM could derive from it. Diagnosis of GD1 is often inaccurate or delayed since symptoms and signs of disease are nonspecific or underhand. We designed a prospective study to investigate the prevalence of GD1 in a large MM patient population to provide substantial evidences to consider GD1 in the pathogenesis and management of MM.
Patients and Methods. This is an observational, prospective, cross-sectional, multicentre study. Twenty-five Italian hematologic centers participated in this study. Due to the lack of data on the effective prevalence of GD1 in MM, the sample size has been determined considering clinically relevant a prevalence of the condition > 0.5% for defining as “high risk” the selected population. Considering an alpha error of 5% and a statistical power of 95%, approximately 1000 patients should beenrolled.. All MM patients are screened by Dried Blood Spots (DBS) sampling technique, that was centralized at the Istituto per la Ricerca e l'Innovazione Biomedica CNR-Palermo. All patients with a positive DBS test undergo genetic test to confirm the diagnosis of GD1. Primary end-point of the study is the prevalence of DBS test positivity in MM patients, which measures glucocerebrosidase activity in peripheral blood leukocytes (normal range 0.2-2.5 nMol/h/m). If the observed prevalence had been significantly higher than predicted, we would have tried to identify the population at higher potential risk of associated GD1.
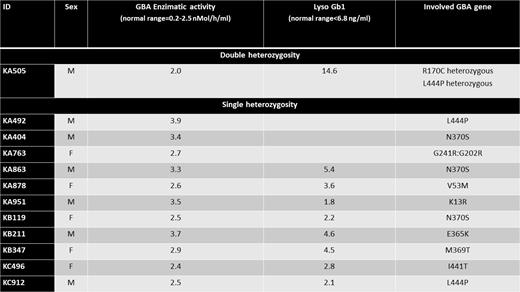
Results. We enrolled 902 patients with a median age of 68 years (range 37-90), 60% of which were male. No patients were Jews, 2 were Asian and 10 were Black, all others were Caucasian. Newly diagnosed and relapsed-refractory MM were 65% and 35%, respectively, whereas SMM and MM were 15% and 85%, respectively. Monoclonal component was IgG in 60%, IgA in 25%, light chain in 15%. Median Ferritin was 310 ng/ml (range 17-1236) and median Alkaline Phosphatase was 75 U/L (range 29-355). Descriptive data of DBS test, enzymatic activity and genetic tests are reported in the Table. In summary, DBS test was found positive in 12/902 (1.33%). Genetic test confirms GBA gene mutations, single heterozygous in 11 patients and double heterozygous in 1 patient. N370S and L444P loci accounted for 50% of GBA gene mutations found. Double-heterozygous patient started replacement therapy for GD1 with imiglucerase.
Conclusions. After the enrolment of more than three quarter of the planned patients, the prevalence of DBS test positivity was higher than 0.5% (1.3%). Genetic test confirmed involvement of GBA genes, all but one in heterozygosity fashion. This study allowed to identify one patient with MM and GD1 requiring therapy for both diseases at the same time. The results of this study support further investigations on the implication of the GD1 in the pathogenesis and management of MM. More data on MM patient subgroups at higher risk of associated GD1 may be provided.
Disclosures
Offidani:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Petrucci:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Celgene-BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Roche: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Menarini: Membership on an entity's Board of Directors or advisory committees. Fazio:Beigene: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; GSK: Membership on an entity's Board of Directors or advisory committees. Kordasti:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Beckman Coulter: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal